Pfizer COVID vaccine
A new study suggests the Pfizer-BioNTech coronavirus vaccine is highly successful at stopping coronavirus infection even six months down the road. For additional information please refer.

Fda Grants Full Approval To Covid 19 Vaccine Developed By Pfizer
Moderna didnt comment when we asked.
. The Pfizer-BioNTech COVID-19 vaccine named BNT162b2 and known as Comirnaty in the European Union is a two-dose mRNA vaccine developed by two pharmaceutical industry. A clinical trial demonstrated that the vaccine has an efficacy rate of over 90 percent in preventing Covid-19. Pfizer-BioNTech COVID-19 Vaccine also known as BNT162b2 This product information is intended only for residents of the United States.
Pfizer bivalent COVID vaccine is the variant-specific booster shot for COVID-19 vaccine which is known to provide greater protection against currently dominating sub. The Pfizer-BioNTech COVID-19 vaccine was authorized for use in Canada under the Interim Order respecting the importation sale and advertising of drugs for use in relation to COVID-19. The updated booster vaccine made by PfizerBioNTech targeting two coronavirus variants has been approved for use in individuals aged 12 years and above.
And BioNTech SE today announced that the US. Pfizer and BioNTech leveraged. Pfizer-BioNTech COVID-19 Vaccine Bivalent is authorized for use to prevent COVID-19 in individuals 12 years of age and older as a single booster dose administered at least 2.
Dose 1 Dose 2. 12 hours agoA Pfizer executive said Monday. Right now Pfizer isnt sharing its vaccines for research purposes a spokesperson confirmed to STAT.
Pfizer-BioNTech COVID-19 Vaccine also known as BNT162b2 This product information is intended only for residents of the United States. Emergency uses of the vaccines have not been approved or licensed by FDA but have been authorized by FDA under an Emergency Use Authorization EUA to prevent Coronavirus. Emergency uses of the vaccines have not.
The Pfizer-BioNTech vaccine is one of several vaccines that can protect against COVID-19. Research indicates that the vaccine has high efficacy in people aged 16 and over. COMIRNATY and the Pfizer-BioNTech COVID-19 Vaccine have the same formulation and are considered interchangeable by Health Canada.
Moderna said it is suing Pfizer and its German partner BioNTech for patent infringement linked to the development of the first Covid-19 vaccines. Producing a batch of the Pfizer-BioNTech vaccine currently takes 60 days. A senior Pfizer executive has admitted that the drug company did not know whether its Covid vaccine prevented transmission of the virus when it began rolling out the shots globally.
Food and Drug Administration FDA has authorized for emergency use a booster dose of the Pfizer-BioNTech COVID-19 Vaccine. The study published in. Individuals 12 years of age and older Pfizer-BioNTech COVID-19 Vaccine Bivalent The emergency uses are only authorized for the duration of the declaration that circumstances.
Systemic reactions in persons aged 55 years Pfizer-BioNTech COVID-19 vaccine and Placebo. BRUSSELSDuring a hearing today on the European Unions COVID-19 response Pfizers president of international developed markets Janine Small admitted that its vaccine. In the fight against COVID-19 a vaccine is a critical part of addressing the global health crisis by decreasing rates of infection disease and death worldwide.
Pfizers stance is legal and in. Prepare and administer the vaccine following manufacturers guidance which is outlined in CDCs Preparation and Administration Summary below.
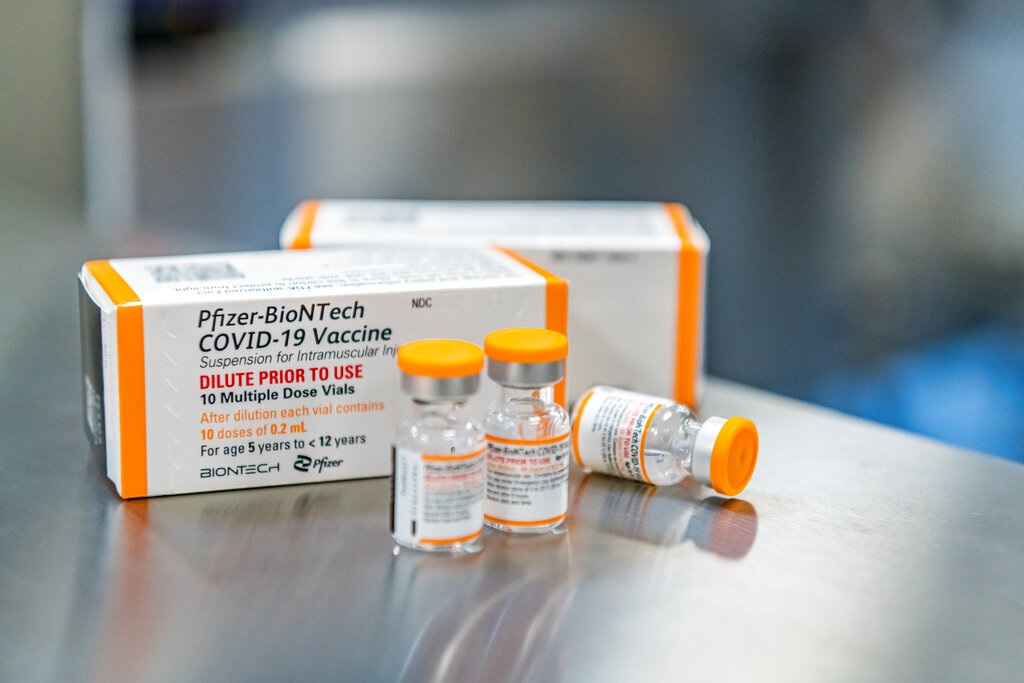
Dph Advises Vaccine Providers To Offer Pfizer Covid 19 Vaccine For Children 5 To 11 Years Of Age State Of Delaware News

Covid 19 Vaccine Storage And Handling Philadelphia Immunization Program

Pfizer Biontech Covid Vaccine Is 73 Effective In Young Children Study Finds

Pfizer Already Agreed To Delay Supply Of Covid 19 Shots To Eu Now The Bloc Wants More
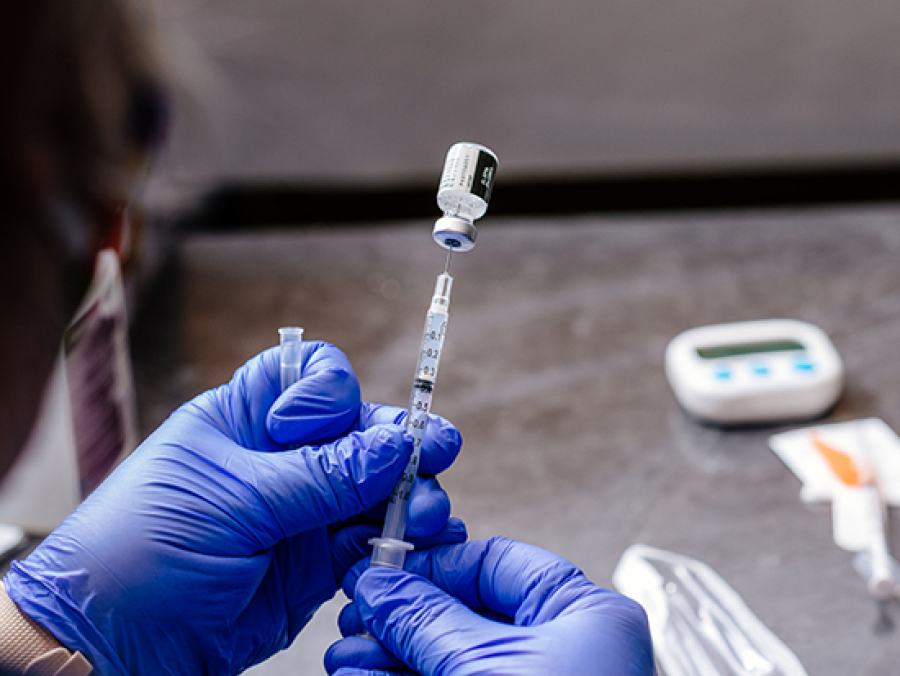
Three Things To Know About The Long Term Side Effects Of Covid Vaccines News Uab

Vaccination For Covid 19 University Health Service

Pfizer Biontech Covid Vaccine Is First To Win U S Authorization Scientific American

Study Pfizer Covid 19 Vaccine Effective With Variant First Detected In U K
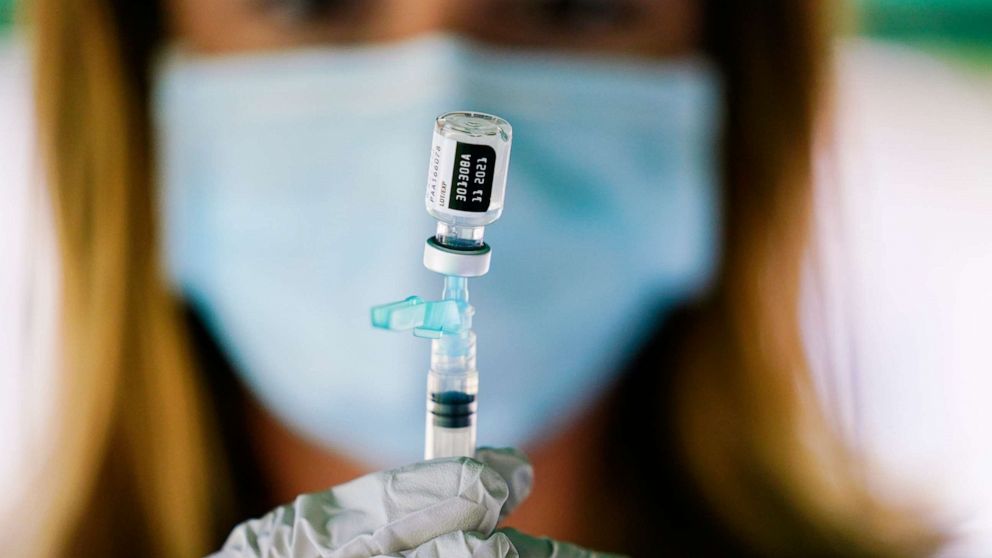
Pfizer Says Covid 19 Vaccine Safe Effective For Kids Ages 5 To 11 Abc News
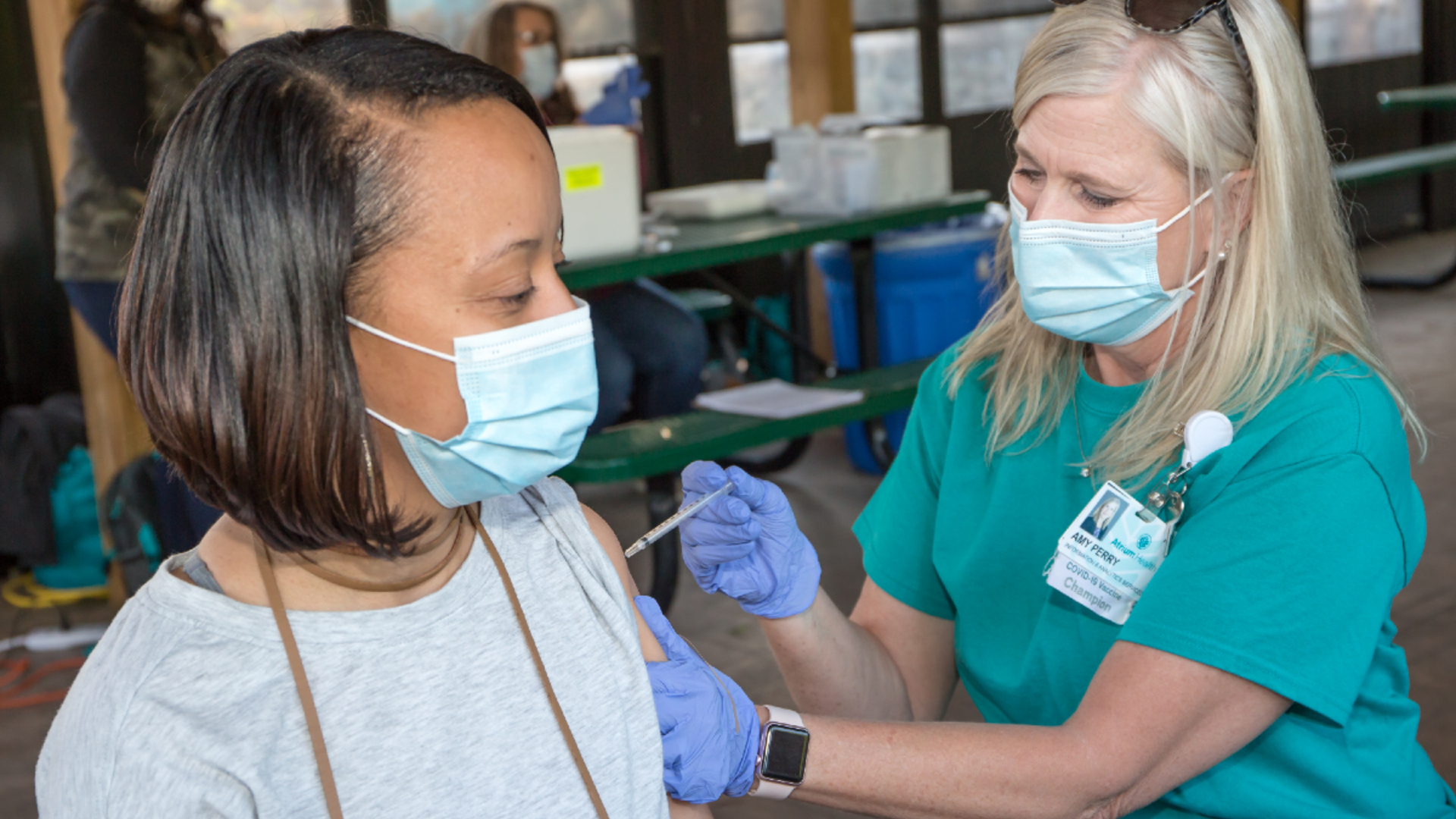
Daily Dose Fda Gives Full Approval Of The Pfizer Vaccine Instilling Confidence For Many

Covid Vaccines Prevented At Least 330 000 Deaths Among U S Seniors In 2021
Pfizer Covid Vaccine Clears Japan Panel For Use With Young Children Reuters
The Fda S Full Approval Of The Pfizer Covid 19 Vaccine What It Means For You Houston Methodist On Health

Data On Pfizer S Covid Vaccine In Teens Published Medpage Today

Pfizer Begins Study Of Covid Shots Updated To Match Omicron Whyy
Bahrain Becomes Second Country To Approve Pfizer Covid 19 Vaccine Coronavirus Pandemic News Al Jazeera